SkinStim Technology
About SkinStim
SkinStim is combination bioelectric stimulation and autologous (from patient to patient) and homologous stem cell, amniotic fluid and skin matrix based therapy for skin regeneration. The company’s patented and patent pending bioelectric signals control with precision the release of SDF-1 a stem cell homing protein. There are additional signals for controlling stem cell proliferation and differentiation into new fresh skin tissue. IGF1, EGF, HGF, Activin A+B, Follistatin and PDGF are expressed via bioelectric signaling and are intended to promote skin regeneration and DNA repair. VEGF, eNOS and HIF1a releases are stimulated for improving blood circulation. But most unique and perhaps most important of all for continued skin health and look is our patent pending bioelectrical signaling sequence for tropoelastin which promotes improved elasticity of skin tissue.
SkinStim treatments are designed to followed by the regulator application use of the proprietary Luminaire facial cream which is chock full of stem cell extracts and skin regeneration promoting growth factors.
The SkinStim team has unmatched experience with autologous stem cell, growth factor and bioelectric organ regeneration solutions. Our team member Dr. Race Kao completed the first stem cell regeneration studies in large animals 1988. In the early 1990’s Dr. Stuart Williams, one of our founding research leaders, first collected stromal fraction, endothelial progenitor cells and stem cells from fat (adipose) tissue for therapeutic purposes. Our team published our first bioelectric regeneration study in 1999. Our team members led human clinical trials applying stem cells to regenerate major organs starting in May 2001 (the heart). All this accumulated knowledge and experience has led to the creation of the SkinStim product platform for skin regeneration.

SkinStim’s bioelectric stimulator OEM manufacturer has FDA 510K market clearance for improving blood circulation and improving muscle/skin tone covering a frequency range inclusive of many of our patented and patent pending bioelectric signals. SkinStim plans to file with the FDA soon amendments requesting permission to add additional signals not found in the original range authorized under the original 510K. This OEM manufacturing team has been producing electrical stimulation devices since 1955 in California.
SkinStim is intended to be used by physicians or other appropriate healthcare providers for homologous uses of skin tissues/integument.
SkinStim”s cells, membranes and matrix scaffolds are regulated by the FDA as an HCT/P – HOMOLOGOUS EXEMPTION RULE – solely under Section 361 of the Public Health Service Act and 21 CFR 1271. The FDA has specific regulations governing HCT/Ps. HCT/Ps that meet the criteria for regulation solely under Section 361 of the Public Health Service Act and 21 CFR 1271 (361 HCT/Ps) are not subject to pre-market clearance or approval requirements, but are subject to post-market regulatory requirements.
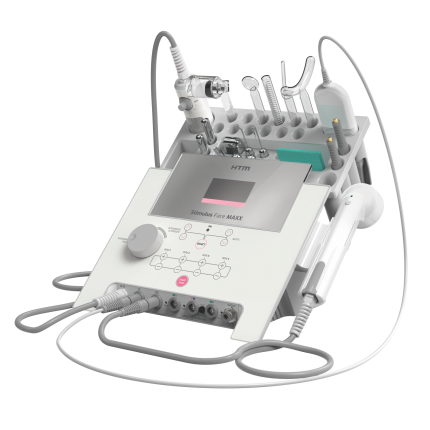
SkinStim Max Simulator Model 440
The SkinStim Max 440 is a facial multi-therapies device, the most complete and versatile multiplatform in the market. The device has 4 output channels and different technologies that allow complete treatments through the resources:
- HighForce current
- Russian current
- Galvanic current
- Microgalvanic current
- MENS current
- Vaccumtherapy
- Ultrasonic peeling
- High frequency
- Phototherapy
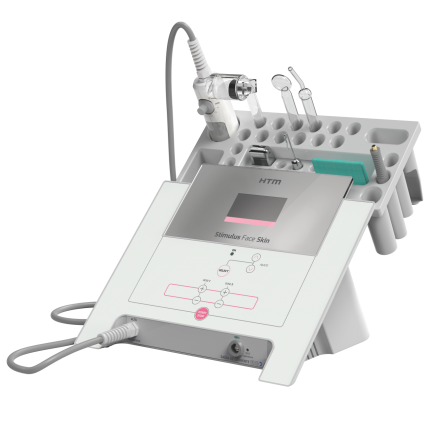
SkinStim Stimulator Model 340

SkinStim Portable Stimulator Model 250-P
The innovative SkinStim Portable Stimulator Model 250-P is the most complete current portable device in the market, that allows a number of esthetical treatments through biphasic and polarized currents:
- TENS
- FES
- High Force
- Russian
- Lipolysis
- Galvanic
- Micro galvanic
- MENS

Poor general medical condition or any pathology that would limit the blood supply and compromise healing, as well as nonvascular surgical sites, should be considered when selecting patients for SkinStim™, as such conditions may compromise successful outcomes or lead to sub-optimal results.
Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures must be taken. Unused or expired tissue product should be discarded according to local, state, federal and institutional requirements. Utilization of the SkinStim’s cell, membrane, growth factor or matrix scaffolds, process and/or technology is limited to healthcare professionals and facilities that are capable of handling such tissue products.
Potential adverse effects may include but are not limited to the following: local tissue, wound bed, regional tissue, or systemic infection, hypersensitive, allergic, or other immune response to the product or trace amounts of antibiotic retained from primary harvest, deleterious effects on potential surrounding or adjacent autologous, allogeneic, or xenogenic grafts, skin substitutes, or other reconstructions including infection and/or failure of adjacent grafted material to take and heal, requirement for further surgical operation(s) and/or debridement, or death.
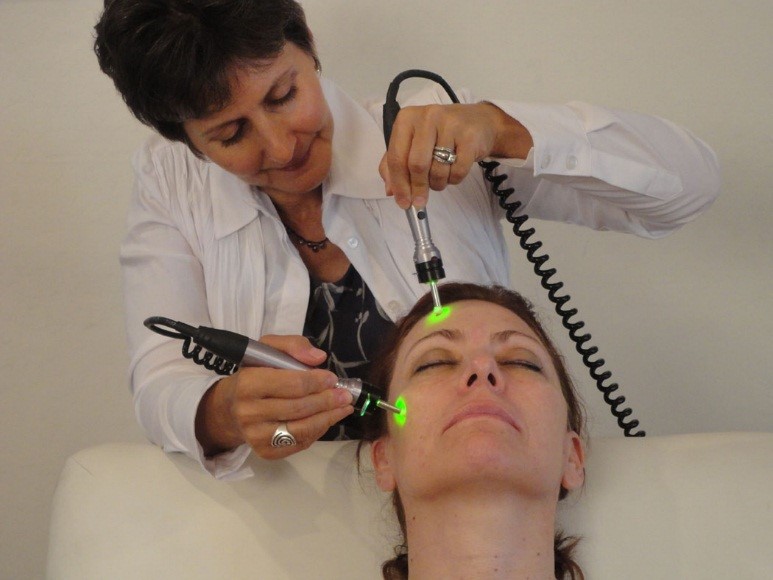
Cosmetic Bioelectric Micro-Current
The SkinStim bioelectric skin regeneration system is a revolutionary method designed to not only tighten and firm Skin but to regenerate it. Our SkinStim proprietary bioelectric signals penetrate deep into the face and is designed to increase production of collagen and elastin which smooth out lines and softens wrinkles. In addition, it is designed to increase absorption of natural nutrients and peptides especially when used with our proprietary Luminaire stem cell extract nutrient hydrogel based skin cream.
The SkinStim bioelectric microcurrent system is designed for improvement in the appearance of the skin.
- Aged and slackened skin.
- Improvement of skin texture
- Fine lines and wrinkles
- Reduction of Acne scars
- Use pre and post-surgery to improve the both muscle and tissue for optimum outcome.
- Post surgically the application of microcurrent supports reduction of trauma, irritation, inflammation and helps foster skin healing as well as minimizing scar tissue.
The Technology behind bioelectric microcurrennt
The use of microcurrent in medicine and cosmetic improvement has been studied for over 30 years. Stimulation with microcurrents is also called bio stimulation or bioelectric therapy because it encourages cell physiology and growth. Essentially, microcurrent is a low level of electrical current that mirrors the natural current flow of the body. It serves as a non-invasive augmentation of the body’s natural electrophysiology through frequency, polarity balancing, and homeostasis. The effects of microcurrent (electroporation) in clinical medicine has demonstrated acceleration of healing bone tissue, wound healing, muscle rehabilitation, TMJ, tendon repairs, lymphedema, diabetes, and collagen remodeling.
In summary bioelectric microcurrent is designed to…
- Recruit a persons own stem cells to their skin with a homing signal SDF-1+PDGF.
- Promote cell metabolism and tissue repair
- Support improved blood circulation.
- Support lymph node health.
- Reduce inflammation
- Diminish lymphedema in cancer patients
- Help increase mitochondria activity through increasing ATP
- Increases natural production of collagen and elastin
- Support scar repair by dispensing scar tissue and collagen remodeling
- Increase protein synthesis, gluconeogenesis (GNG) and membrane transport
- Reeducate and rejuvenate muscle tissue
- Support healing of bone
- Heal skin ulcerations
Leonhardt Ventures and SkinStim History
- 2018 – Chris Rosgen formerly of BioLase and Nugene hired as President of SkinStim.
- 2017 – Leonhardt founded Second Heart Assist raises $1 million and completes 4 large animal studies.
- 2016 – Grant support received for entering BioInnovations Gateway USTAR lab in Utah.
- 2016 – Grants received for eye and heart regeneration studies in Utah.
- 2016 – 30 startups are in Leonhardt’s Launchpads accelerator(s) in California and Utah. 26 based on same core technology platform of bioelectric + micro pump + composition for organ regeneration.
- 2015 – Leonhardt team opens up accelerator in Utah.
- 2015 – Leonhardt founded Vascustim (formerly MyoStim Peripheral) completes 8 patient pilot wound healing study in Mexico with bioelectric stimulation.
- 2013 – Leonhardt team opens up accelerator in Los Angeles.
- 2012 – Leonhardt led team completes 16 patient limb salvage study in Czech Republic utilizing adipose derived cells.
- 2012 – Leonhardt founds BioLeonhardt to focus on bioelectric stimulation + a micro infusion pump + a fifteen component stem cell based composition for heart regeneration.
- 2008 – Leonhardt moves to California and founds innovation and startup accelerator Leonhardt’s Launchpads focused on combining bioelectrics, stem cells, support factors and micro infusion pump for organ regeneration opening first in Northern California on the campus of the University of Northern California.
- 2008 – Bioheart, Inc. goes public on NASDAQ team completes Pilot, Phase I, Phase II and Phase II/III interim studies. Raised over $145 million in total since founding.
- 2005 – Leonhardt Vineyards LLC DBA Leonhardt Ventures formed in California.
- 2001 – Leonhardt team completes historic first ever non-surgical stem cell repair of a human heart in Rotterdam, The Netherlands.
- 2000 – Leonhardt files first of series of bioelectric organ regeneration patents.
- 1999 – Dr. Shinichi Kanno of Leonhardt team published first paper on bioelectric driven healing and regeneration.
- 1999 – Leonhardt forms Bioheart, Inc. first stem cell organ regeneration company (heart).
- 1998 – AVE merger closes and Medtronic acquires combined company.
- 1997 – World Medical Mfg. Corp. receives first offer to merge with AVE.
- 1995 – Leonhardt team completes historic first ever percutaneous repair of an aortic aneurysm in Melbourne, Australia.
- 1994 – Leonhardt’s Launchpads co-founder Dr. Stuart Williams develops and patent first devices to harvest cells from fat tissue. Leonhardt team begins collaboration with him at U of Arizona to cell seed or sod stent grafts.
- 1991 – Leonhardt team develops and later patents first commercially successful stent graft for aneurysm repair.
- 1991 – Leonhardt team develops first percutaneous heart valve StentValve ands first catheter heart valve decalcification device Valvublator.
- 1990 – Leonhardt team completes $300,000 investment deal with Nippon Zeon Co. of Japan for PolyCath.
- 1990 – Leonhardt team gets FDA 510K for cardiovascular balloon catheters including 72 hour implanted versions.
- 1989 – Leonhardt team partners with Labor and DMG in Brazil for heart valves and oxygenators.
- 1989 – Leonhardt files first series of patents for predictably compliant cardiovascular balloon catheters.
- 1988 – Leonhardt team develops and later patents first stem cell delivery catheter for deep organs ProCell.
- 1988 – Team lead by Dr. Race Kao completes first large animal studies of stem cell based regeneration (hearts).
- 1988 – World Medical Manufacturing Corp. formed by Leonhardt.
- 1986 – World Medical Corporation formed by Leonhardt.
- 1985 – Howard Leonhardt reads The Body Electric and contacts author Dr. Robert. O. Becker
- 1985 – Howard Leonhardt joins International Marketing Advisors
- 1983 – Howard Leonhardt Joins American Generation Medical Corporation as first employee.
- 1982 – HJ Leonhardt & Co. formed.

