Skinstim Product Catalog
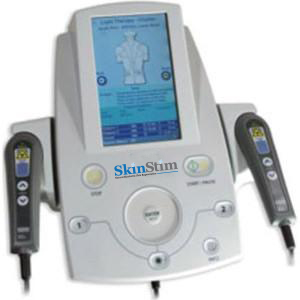
SkinStim Model 240
High Precision Bioelectric and TENS Stimulator
Pre-Programmed for SDF1, VEGF, IGF1 and Tropoelastin Controlled Release
FDA 510K Market Cleared
Retail Price: $5,995

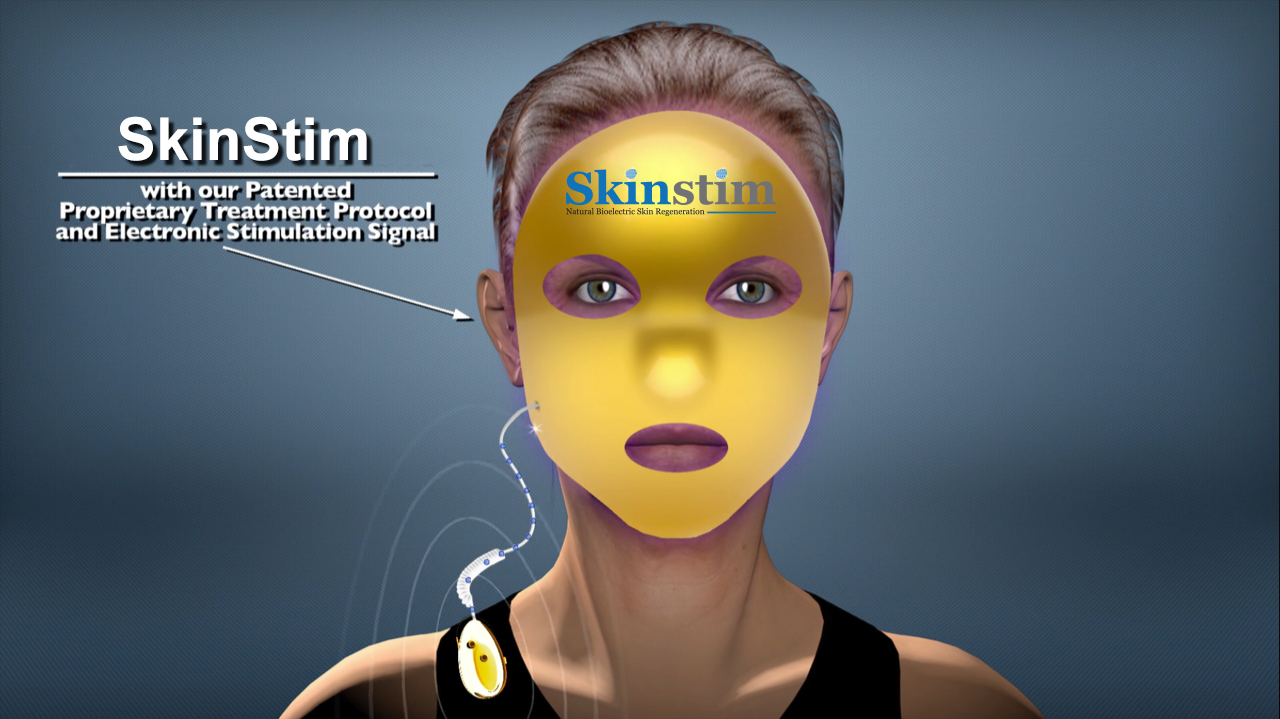
SkinStim Model 100
Microcurrent and LED Face Mask
FDA 510K Market Cleared
Retail Price: $350

Microcurent and LED Mini-Mask Model 200
Microcurrent Mini Face Mask
FDA 510K Market Cleared
Retail Price: $300

SkinStim EyeMask Model 100
Microcurrent Eye Mask
FDA 510K Market Cleared
Retail Price: $100

SkinStim Gel Electrodes
Microcurrent Gel Electrodes
FDA 510K Market Cleared
Retail Price: $15.00
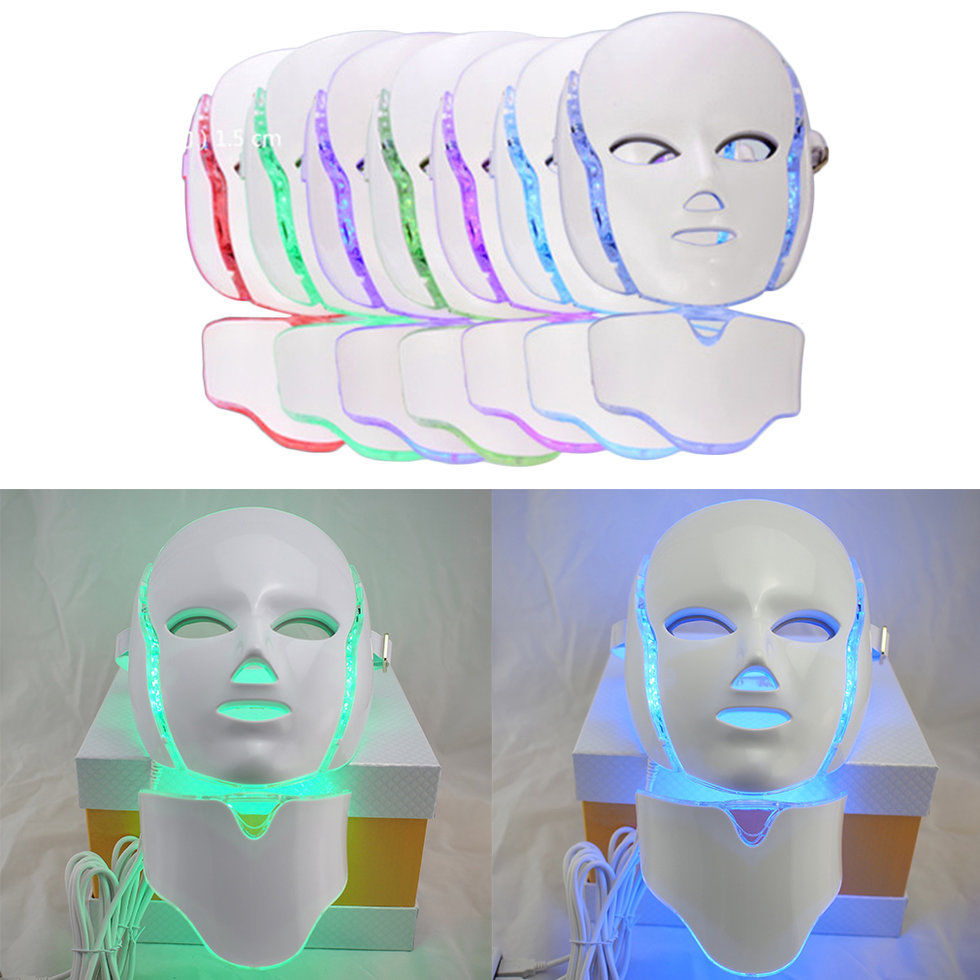
LED Light and Bioelectric Therapy Combination Face Mask with Neck Piece Add On
Price $400 with neck add on

SkinStim GosearTM
Electroacupuncture Pen
FDA 510K Market Cleared
Retail Price: $50

SkinStim AxoBio
Room Temperature Amniotic Fluid
Produced in FDA Certified cGMP Facility in Arizona
Retail Price: $5000 per 2ml Vial
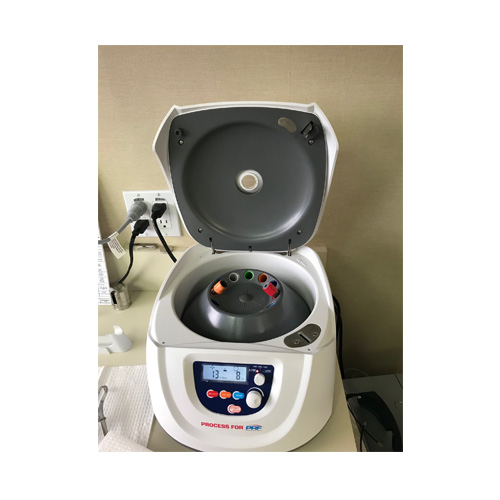
SkinStim Bedside PRF Device
Platlet Rich Fibrin Processing Device
FDA 510K Market Cleared
Retail Price: $7500

Hydrogel Skin Mask
Retail Price: $10.00

Collagen Face Mask
Retail Price: $50.00

DermaPenTM
Microneedle Array
FDA 510K Cleared
Retail Price: $3900
SkinStim Microcurrent + Light Therapy
Non-invasive Product Development

FACE MASK (CONDUCTIVE)
CONDUCTIVE FACE MASK
SkinStim Consumer Product
Notice: Product sales are subject to strict conditions including operator training certification. All marketing, labeling and use must be in full compliance with U.S. 510K authorized indications of use in the U.S.A (see below links) and CE Mark authorization outside of the USA. Any use of the product outside of these authorized indications of use MUST be via an authorized/approved IDE Investigational Device Exemption clinical study with the FDA in the USA or applicable equivalent regulatory filings and authorizations outside of the USA, with proper patient consent forms, appropriate monitoring controls and Institutional Review Board/Ethics Committee approval and oversight.
See Mettler 240 FDA 510(k) Number: (K031077) Application link below for authorized labeling and indications of use and also full listing of all 510K applications filed to date on the electrical stimulator product line manufactured for SkinStim by Mettler Electronics of Anaheim, California.
Mettler 240 FDA 510(k)
Search Results
[PDF]Mettler SYs*Stim 240, ME 240 – FDA
510(k) SUMMARY-. Mettler SYs*Stim 240, ME 240. Submitter’s Name: Mettler Electronics Corp. Address: 1333 South Claudina Street. Anaheim, CA 92805.
- ” Relaxation of muscle spasms
- ” Prevention or retardation of disuse atrophy
- ” Increase local blood circulation
- * Muscle re-education
- ” Maintaining or increasing range of motion
- ” Immediate post surgical stimulation of calf muscles to prevent venous thrombosis
e Post-surgical acute pain
-
* Temporary increase in local blood circulation
-
* Temporary relief of minor muscle and joint aches, pains and stiffness
-
* Relaxation of muscles
-
* Temporary relief of Muscle spasms
-
* Temporary relief of minor pain and stiffness associated with arthritis
SYS*STIM 240 – 510(k) Premarket Notification
FDA 510(k) Applications Submitted by METTLER ELECTRONICS …
510k.medevnet.com/…/index.cfm?…METTLER%20ELECTRONICS%20CORP%2E
K111482 · 05/27/2011 · SONICATOR PLUS 920 · METTLER ELECTRONICS CORP. K113017 · 12/05/2011 · SYS*STIM 240 · METTLER ELECTRONICS CORP.
